 |
There is a PDF file for download
an unsaturated hydrocarbon alkene for high school
There are exercises and answers
|
អ៊ីដ្រូកាបួមិនឆ្អែត អាល់សែន មេរៀន Update ថ្មីរួមមានៈ
An Unsaturated Hydrocarbon alkene Last updated
Physical properties
Many of the physical properties of alkenes and alkanes are similar: they are colourless, nonpolar, and combustable. The physical state depends on molecular mass: like the corresponding saturated hydrocarbons, the simplest alkenes, ethene, propene, and butene are gases at room temperature. Linear alkenes of approximately five to sixteen carbons are liquids, and higher alkenes are waxy solids. The melting point of the solids also increases with increase in molecular mass.
Chemical properties
Alkenes are relatively stable compounds, but are more reactive than alkanes, either because of the reactivity of the carbon–carbon pi-bond or the presence of allylic CH centers. Most reactions of alkenes involve additions to this pi bond, forming new single bonds. Alkenes serve as a feedstock for the petrochemical industry because they can participate in a wide variety of reactions, prominently polymerization and alkylation.
Addition reactions
Alkenes react in many addition reactions, which occur by opening up the double-bond. Most of these addition reactions follow the mechanism of electrophilic addition. Examples are hydrohalogenation, halogenation, halohydrin formation, oxymercuration, hydroboration, dichlorocarbene addition, Simmons–Smith reaction, catalytic hydrogenation, epoxidation, radical polymerization and hydroxylation.
Hydrogenation
Hydrogenation of alkenes produces the corresponding alkanes. The reaction is carried out under pressure at a temperature of 200 °C in the presence of a metallic catalyst. Common industrial catalysts are based on platinum, nickel or palladium. For laboratory syntheses, Raney nickel (an alloy of nickel and aluminium) is often employed. The simplest example of this reaction is the catalytic hydrogenation of ethylene to yield ethane:
Hydration
Hydration, the addition of water across the double bond of alkenes, yields alcohols. The reaction is catalyzed by strong acids such as sulfuric acid. This reaction is carried out on an industrial scale to produce ethanol.
Alkenes can also be converted into alcohols via the oxymercuration–demercuration reaction , the hydroboration–oxidation reaction or by Mukaiyama hydration.
Halogenation
In electrophilic halogenation the addition of elemental bromine or chlorine to alkenes yields vicinal dibromo- and dichloroalkanes (1,2-dihalides or ethylene dihalides), respectively. The decoloration of a solution of bromine in water is an analytical test for the presence of alkenes:
Related reactions are also used as quantitative measures of unsaturation, expressed as the bromine number and iodine number of a compound or mixture.
Hydrohalogenation
Hydrohalogenation is the addition of hydrogen halides such as HCl or HI to alkenes to yield the corresponding haloalkanes:
If the two carbon atoms at the double bond are linked to a different number of hydrogen atoms, the halogen is found preferentially at the carbon with fewer hydrogen substituents. This patterns is known as Markovnikov's rule. The use of radical initiators or other compounds can lead to the opposite product result. Hydrobromic acid in particular is prone to forming radicals in the presence of various impurities or even atmospheric oxygen, leading to the reversal of the Markovnikov result:
|
ឯកសារនេះ រៀបចំឡើងក្នុងគោលបំណង ពង្រឹងបន្ថែមនូវសមត្ថភាព និងជាមូលដ្ឋានគ្រឹះក្នុងការសិក្សាកាន់តែស៊ីជម្រៅ និងច្បាស់លាស់បន្ថែមទៀតទៅលើមេរៀនអាល់សែន ។ ការដក់ស្រង់សំរាំងពិសេសៗ យកគន្លឹះល្អៗ មើលហើយ ងាយយល់ឆាប់ចាប់បាន ។ ថែមទាំងមានការរចនាពណ៍យ៉ាងល្អប្រណិត មើលហើយធានាថាត្រជាក់ភ្នែក ហេហេ។ ម្យ៉ាងទៀតការរៀបរៀងនិងចងក្រងឡើង ទុកជាឯកសារនិងចែករំលែកដល់បងប្អូន សិស្សានុសិស្ស និស្សិត ដែលមានបំណងចង់យល់កាន់តែច្បាស់ពី មុខវិជ្ជាគីមីសរីរាង្គអាល់សែន។ ជាពិសេសជាងនេះទៅទៀតនោះ ខ្លឹមសារចំនុចនិមួយៗមានលក្ខណៈពន្យល់ច្បាស់លាស់ល្អ ដែលអាចបំពេញបន្ថែមនូវសមត្ថភាព និងចំនេះដឹងខាងមុខវិជ្ជាគីមីសរីរាង្គអាល់សែន សម្រាប់ជាមូលដ្ឋានគ្រឹះក្នុងការប្រឡងនានា។ ជារួមមក បើទោះបីការខិតខំរៀបរៀងវិញ្ញាសាមួយនេះយ៉ាងម៉ត់ចត់ក៏ដោយក៏នៅតែមានចំនុចខ្វះខាតជាក់ជាមិនខានឡើយ ទាំងការសរសេរ ទាំងអត្តន័យ និងទាំងដំណោះស្រាយ ហើយរាល់ចំនុចខ្វះខាតទាំងនោះ ខ្ញុំបាទរីករាយនឹងទទួលយក នូវការជួយកែលំអពីប្រិយ៍មិត្តគ្រប់មជ្ឈដ្ឋានសិក្សា។ ដូច្នេះហើយ ប្រសិនបើមានកង្វះខាត ឬខុសឆ្គងត្រង់ចំនុចណាមួយដោយអចេតនានោះ ខ្ញុំសូមមិត្តអ្នកអានមេត្តាអធ្យាស្រ័យ និងជួយផ្ដល់ជាយោបល់។
This document is designed to strengthen the capacity and fundamentals of more in-depth and clear study of alkene lessons. Special extra quotes, good tips, and easy to understand, get a lot of attention. Also have a nice design for look and make sure with cool eyes hahaha.... In addition, the composition and compilation are documented and shared to students, students who wish to better understand the alkene organic matter. More specifically, each topic has a well-defined explanation, which complements the capability and knowledge of the alkene organic matter as the foundation of the exam. In essence, despite the rigorous set of disciplines, there are still a lot of bottlenecks in both writing, both in terms of both meaning and solution, and I am happy to receive help. Improve all friends from all walks of life. Therefore, if there is any mistake or mistake at some point, I would like to ask readers to be kind and tolerate. Thanks for all the feedback in the constructive sense.
ខ្ញុំបាទសូមអរគុណរាល់មតិរិះគុណទាំងឡាយណាក្នុងន័យស្ថាបនា។


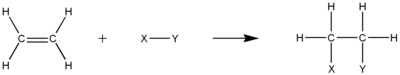




អរគុណណាស់សម្រាប់ការចូលមើល
ReplyDelete